



Related Attributes
Product details
Actinomycin D powder A bright red crystalline powder that is hygroscopic and gradually loses its activity under light exposure. Insoluble in petroleum ether, slightly soluble in water, soluble in methanol, ethanol and ethyl acetate, easily soluble in benzene, chloroform and acetone. Actinomycin D CAS 50-76-0 is effective in the treatment of malignant mole, nephroblastoma, Hodgkin's disease and testicular tumor. Cervical cancer, ovarian cancer, breast cancer and digestive tract cancer, etc., also has a certain effect. Actinomycin D Raw Materials combined with radiotherapy can improve the sensitivity of radiotherapy.
What symptoms can actinomycin D be used to relieve?
·Inhibit the proliferation of miasma cells,
Enhance the therapeutic effect of radiotherapy.
Immunosuppressive effect.

Effects and efficacy.
Actinomycin D, an antitumor antibiotic, is commonly used in the form of injection. It has outstanding effects on Hodgkin's disease (HD) and neuroblastoma, especially in controlling fever. It is often used in combination with other drugs to treat gestational trophoblastic tumors and other solid tumors (brain tumors, nephroblastomas and various sarcomas)
Applications/Functions of Actinomycin D powder.

What diseases can actinomycin D be used to treat?
·This product has outstanding effects on Hodgkin's disease (HD) and neuroblastoma, especially in controlling fever.
·For the initial treatment of non-metastatic varicella, the cure rate is 90%~100%, which is similar to the effect of methotrexate (MTX) alone.
·It is also effective for testicular cancer and is generally used in combination with other drugs.
Physicochemical Property of Actinomycin D powder
Bright red crystalline powder, hygroscopic, gradually loses activity under light. Actinomycin D CAS 50-76-0 insoluble in petroleum ethers, slightly soluble in water, soluble in methanol, ethanol and ethyl acetate, soluble in benzene, chloroform and acetone.
Production methodprocess of Actinomycin D powder
Actinomycin D Raw Materials were prepared from culture medium of Streptomyces melaninogenes by extraction, acid alumina column chromatography, vacuum concentration and recrystallization of petroleum ether.

FAQ.
Q: What is the difference between Actinomycin D and Dactinomycin?
A: They are the same compound; "Dactinomycin" is the clinical name.
Q: Can Actinomycin D be used in vitro?
A: Yes, it is commonly used in cell culture studies at controlled concentrations.
Q: What are the alternatives to Actinomycin D?
A: Doxorubicin and Mitomycin C are other DNA-intercalating agents, but with different efficacy profiles.
Product Safety and Compliance Details
Safe use
As the Food and Drug Administration (FDA) advises, dietary supplements can support your overall health but may also have powerful effects on the body. It’s important to read labels carefully, exercise caution, and consult your healthcare professional before taking any supplement. Side effects are more likely if supplements are taken in high doses, as substitutes for prescribed medications, or in combination with multiple supplements. If you experience severe side effects, discontinue use immediately and seek medical attention.
Our advantage.
Henrikang have capabilities to manufacture any capsules formula. From sourcing each ingredient in your formula, to post filling inspection; we do it all at the best prices and the fastest lead times. We have the experience to help you formulate a new product for your target audience, or discuss with you how to properly scale your manufacturing. As your partner, it is our job for long-term capsules manufacturing success.
Make sure to check out our products. There, you can see what we believe is some of the best formulas that are on a level of their own. They possibly offer a wide variety of vitamins and nutrients that may be very beneficial. The trademarks of a good capsules supplement include taste, color, shape, size, and more. We carefully weighed these factors and have put together a catalog of some of the very best formulas we offer for you today.
Why choose us?

HRK Factory

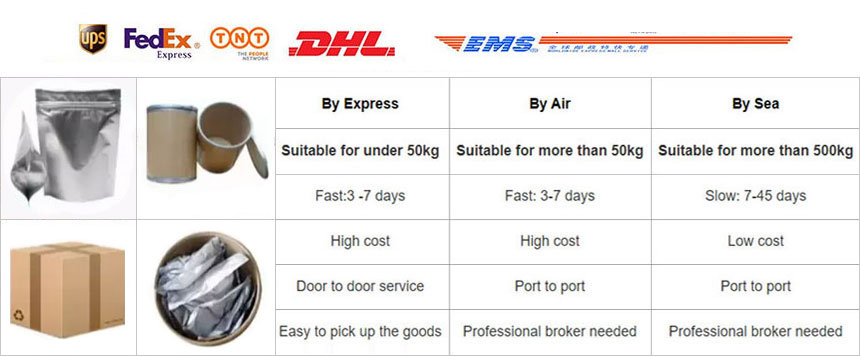
About Shipping
Pharmaceutical Intermediate manufacturers
©2023 Xi'an Henrikang Biotech Co., Ltd.,